Why does beer fizz? This question might pop into your mind while sipping a refreshing brew. The answer lies in beer carbonation, a key process that gives beer its effervescence and significantly impacts its flavor, mouthfeel, and overall drinking experience.
Beer carbonation adds carbon dioxide (CO2) to beer, creating familiar bubbles that make beer lively and enjoyable. But why is carbonation so important?
Let’s dive into the details and discover why this fizzy phenomenon is vital to beer lovers everywhere.
What is Beer Carbonation?
Beer carbonation is the process of dissolving carbon dioxide into beer under pressure. This process is what gives beer its characteristic fizz and refreshing sensation.
Carbonation is vital in beer. It improves the beer’s taste, contributes to its mouthfeel, and affects its appearance.
When you sip a well-carbonated beer, the bubbles dance on your tongue, releasing flavors and aromas that elevate the drinking experience. Beer would be flat, dull, and less enjoyable without proper carbonation.
The bubbles you see in beer are more than just a visual treat; they also play a crucial role in the taste and feel of the beverage. These bubbles help release the aromas integral to the flavor profile of different beers, from craft beer to commercial brews.
The level of carbonation can vary depending on the style of beer. Some styles, like stouts, have lower carbonation, while others, like draft beer, are more highly carbonated to enrich their light and crisp characteristics.
The Science Behind the Bubbles
Understanding the science behind how beer is carbonated can make you appreciate your drink even more. Let’s break down the process.
1. Carbon Dioxide and Pressure
The interaction between carbon dioxide and pressure is at the heart of beer carbonation. When CO2 is introduced into beer, it dissolves under pressure, creating carbonic acid. This acid gives beer a slight tang and contributes to the flavor profile.
The CO2 remains dissolved in the beer if kept under pressure in a sealed container, like a bottle or keg. The pressure is released once the container is opened, and the CO2 escapes from the beer in bubbles.
This is why freshly poured draft beer has that satisfying fizz—it releases CO2 from the liquid into the air.
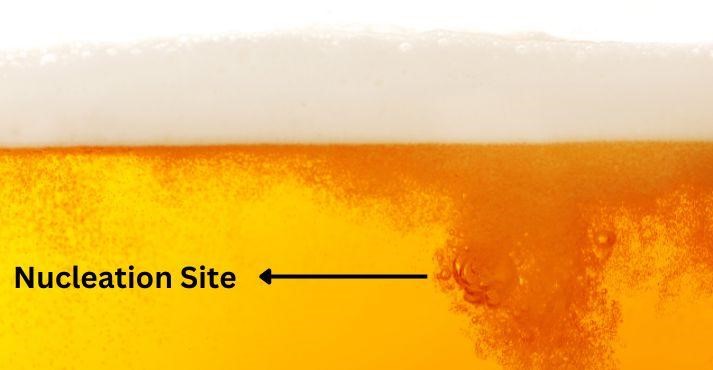
2. Nucleation Sites
But the story of beer carbonation continues after CO2 and pressure. Nucleation sites also influence the formation of bubbles. These are tiny imperfections or particles within the beer, such as small bits of yeast or microscopic scratches in the glass.
Nucleation sites act as the starting points for CO2 to escape from the liquid, forming bubbles that rise to the surface. Without these nucleation sites, how does beer get carbonated and form bubbles?
The beer would remain flat and lifeless. This process is particularly evident when pouring craft beer into a glass with tiny imperfections, where you can see bubbles streaming up from specific spots.
3. The Formation of Bubbles
As the CO2 escapes from the liquid, bubbles rise to the surface, creating the foam, or “head,” that beer is known for. The size and speed of the bubbles can vary depending on the beer type and carbonation level.
For instance, highly carbonated beers like certain ales will have larger, faster bubbles, while beers with lower carbonation, like stouts, will produce smaller, slower bubbles.
Brewers must understand how to carbonate beer, as the level of carbonation directly impacts the final product’s texture and drinkability.
The balance of carbonation is critical. Too much CO2 can result in an over-carbonated beer, which can cause excessive foaming and an overly sharp mouthfeel. On the other hand, under-carbonation can lead to a flat, unappealing beer that needs more character.
Therefore, mastering the science of carbonation is a key skill for homebrewers and professional beer manufacturers alike.
Natural Carbonation Methods
Two primary methods of naturally carbonating beer are bottle conditioning and keg conditioning. Each method has its advantages and imparts unique characteristics to the beer.
1. Bottle Conditioning
Bottle conditioning is a centuries-old method of carbonating beer. This process involves adding a small amount of sugar and yeast to the beer before it is sealed in a bottle.
The yeast ferments the sugar, producing CO2 as a byproduct. Since the bottle is sealed, the CO2 cannot escape and is forced to dissolve into the beer, creating carbonation.
This method carbonates the beer and contributes to its flavor profile as the yeast continues to work in the bottle.
This method is commonly used in craft beer production, emphasizing traditional brewing techniques.
Bottle conditioning requires careful control of sugar added, as too much can lead to over-carbonation and potential bottle explosions.
Conversely, too little sugar can result in under-carbonation, leaving the beer flat. The process also takes time, as the beer needs to sit for several weeks to achieve the desired level of carbonation.
2. Keg Conditioning
Keg conditioning is like bottle conditioning but is done on a larger scale. In this method, the beer is placed in a keg with a small amount of sugar and yeast. The yeast ferments the sugar, producing CO2 that dissolves into the beer, carbonating it naturally.
The main difference between keg conditioning and bottle conditioning is the scale—keg conditioning is often used by larger breweries or for draft beer systems where beer is served directly from the keg.
Keg-conditioned beers tend to have a fresher taste than bottle-conditioned beers, as they are less likely to be exposed to oxygen. Additionally, keg conditioning allows for easier adjustments if the beer is under or over-carbonated, making it a more flexible method for brewers.
Forced Carbonation Methods

Forced carbonation involves directly adding CO2 to the beer rather than relying on natural fermentation processes. This method is quicker and more controlled, making it popular among commercial breweries and food manufacturers.
1. CO2 Injection
One of the most common methods of forced carbonation is CO2 injection. In this process, beer is placed in a sealed container, such as a keg, and CO2 is added under pressure.
The gas dissolves into the beer quickly, allowing for precise control over the level of carbonation. This method is highly efficient and is commonly used in commercial brewing because it allows for consistent carbonation levels across large batches.
CO2 injection is also used in homebrewing, particularly when brewers want to carbonate their beer quickly.
The process involves attaching a CO2 tank to the keg and adjusting the pressure to the desired level. The CO2 dissolves into the beer faster by agitating the keg, achieving carbonation in hours rather than weeks.
This method is ideal for brewers who need to carbonate their beer on a tight schedule or those producing large volumes of craft beer for distribution.
2. Carbonation Stone
Another method of forced carbonation involves the use of a carbonation stone. This porous stone is placed inside a beer container, such as a keg. CO2 is pumped through the stone, which has tiny holes that create beautiful bubbles. These small bubbles dissolve more easily in the beer, producing smoother carbonation.
Carbonation stones are often used in professional brewing setups because they allow for better control over the carbonation process, producing a finer and more consistent level of carbonation.
Using a carbonation stone requires careful monitoring of the CO2 pressure and the beer’s temperature.
The process can take several hours to a few days, depending on the desired level of carbonation. This method is particularly popular for beers that require delicate and even carbonation, such as certain types of craft beer.
3. Kegging
Kegging is another popular method for carbonating beer. In this process, beer is placed in a keg, and CO2 is added under pressure.
The beer is then agitated to help the gas dissolve more quickly. Kegging is a highly effective method for achieving consistent carbonation and is often used in home and commercial brewing.
Kegging also allows for easy adjustments if the beer is under or over-carbonated, making it a flexible option for brewers. This method is particularly common in the production of draft beer, where maintaining a consistent level of carbonation is essential for quality and taste.
Common Issues in Carbonation and How to Fix Them

While carbonation is a key part of beer-making, it can sometimes go wrong. Here’s how to address common carbonation issues, ensuring your beer is as enjoyable as possible.
1. Over-Carbonation
Over-carbonated beer can be a real problem, leading to excessive foaming, a harsh mouthfeel, and potential messes when opening bottles. Over-carbonation is usually caused by adding too much sugar during bottle conditioning or applying too much CO2 in forced carbonation.
The excess CO2 creates a higher pressure within the beer, causing it to foam excessively when opened. This can be particularly problematic for craft beer producers who bottle their beers for sale or distribution.
To fix over-carbonation, you can release some gas by carefully opening and resealing the container. If the beer is already bottled, you may need to let it sit for a while to allow the excess CO2 to dissipate naturally.
Alternatively, transferring the beer to a keg and adjusting the CO2 pressure can help reduce over-carbonation.
2. Under-Carbonation
On the other hand, under-carbonation occurs when there’s not enough CO2 in the beer, resulting in a flat taste and lack of fizz.
This can happen if the yeast doesn’t ferment all the sugar in bottle conditioning or if not enough CO2 is added during forced carbonation. A flat beer is often unappealing, as it needs the refreshing quality of carbonation.
To fix under-carbonation, you can add more sugar and yeast if you’re bottle conditioning, although this method requires careful measurement to avoid over-carbonation.
If you’re using forced carbonation, increasing the CO2 pressure and allowing the beer to sit for a longer period can help achieve the desired level of carbonation. Consistent quality control is essential for food distributors and commercial brewers to avoid under-carbonation in large batches of beer.
3. Foaming Issues
Foaming is another common issue in beer carbonation, often due to improper pouring or excessive carbonation. Pouring beer too quickly or from too high above the glass can cause the CO2 to escape too rapidly, creating a large head of foam.
In addition, using dirty or warm glasses can exacerbate foaming, as these conditions create additional nucleation sites for CO2 to escape.
To reduce foaming, pour the beer at an angle and avoid too much agitation. Chilling the glasses and ensuring they are clean can also help minimize foaming. If foaming is due to over-carbonation, letting the beer sit for a bit before serving can help.
Understanding why beer is carbonated and how to manage it properly is critical to avoiding these issues and ensuring a smooth, enjoyable drinking experience.
Role of Carbonation in Beer Trends
The beer industry constantly evolves, and carbonation plays a significant role in some of the latest trends. For example, the rise of gluten-free beers has prompted brewers to experiment with different carbonation levels to increase the drinkability of beers made without traditional grains.
Similarly, the growing popularity of craft beer has led to a revival of interest in traditional carbonation methods like bottle conditioning and keg conditioning, as these methods are often seen as more authentic and artisanal.
Commercial and local breweries also pay close attention to carbonation trends as consumer preferences shift. For example, lower-carbonation beers are becoming more popular among those who prefer a smoother, less fizzy drink.
On the other hand, highly carbonated beers are still in demand among fans of draft beer, where higher levels of CO2 enrich the crisp, refreshing qualities of the beer.
Understanding these trends and the role of carbonation in beer production can help brewers and food processors stay ahead of consumer preferences and continue to produce innovative and enjoyable beers.
Conclusion
Beer carbonation is an essential part of what makes beer so enjoyable. Whether achieved through natural methods like bottle or keg conditioning or through forced methods like CO2 injection and carbonation stones, the result is a drink that is lively, flavorful, and refreshing.
Understanding the science behind carbonation in beer and how to fix common issues ensures that every sip is as satisfying as possible. The importance of carbonation extends beyond just the bubbles—it plays a crucial role in beer’s taste, mouthfeel, and overall drinking experience.
As the beer industry continues to evolve, with trends like gluten-free beers and craft beer’s resurgence, carbonation’s role remains as important as ever.
Whether you’re a homebrewer, a professional beer manufacturer, or a beer enthusiast, understanding how to carbonate beer and the impact of carbonation on beer quality is vital to enjoying and creating the perfect brew.





























